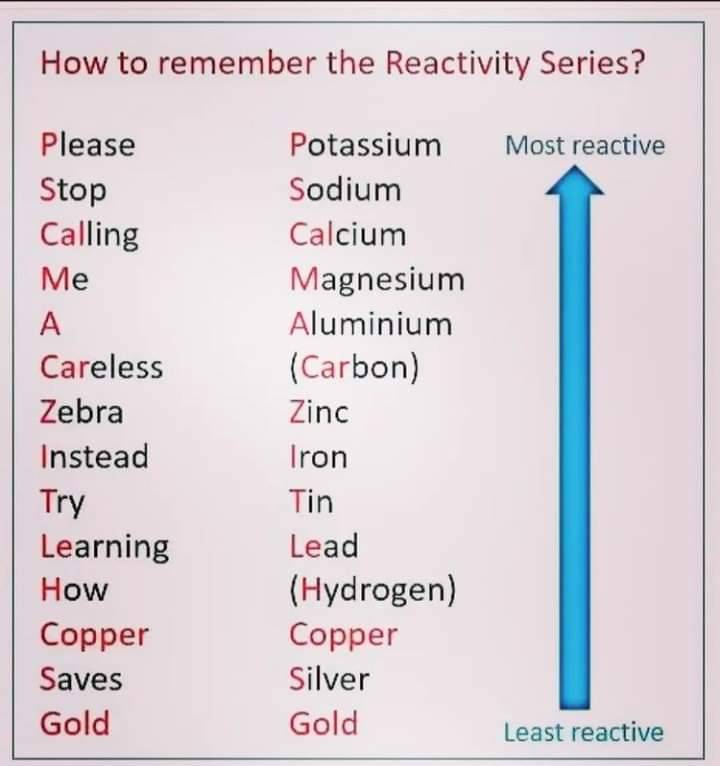
Which Metal Is More Active Than H2 . A reactivity or activity series is the arrangement of metals from most reactive to. Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. Many transition metals such as iron, chromium, nickel, tin, zinc, and lead are more reactive than hydrogen. The activity series is a list of elements in decreasing order of their reactivity. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the. The most active metals are so reactive that they readily combine with the o 2 and h 2 o vapor in the atmosphere and are therefore stored under an inert liquid, such as mineral oil. Since metals replace other metals, while nonmetals replace. 26 rows metals that require the loss of only one electron to form stable ions are more reactive than similar metals which require the loss of.
from svpwiki.com
Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the. A reactivity or activity series is the arrangement of metals from most reactive to. Many transition metals such as iron, chromium, nickel, tin, zinc, and lead are more reactive than hydrogen. Since metals replace other metals, while nonmetals replace. The most active metals are so reactive that they readily combine with the o 2 and h 2 o vapor in the atmosphere and are therefore stored under an inert liquid, such as mineral oil. Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. 26 rows metals that require the loss of only one electron to form stable ions are more reactive than similar metals which require the loss of. The activity series is a list of elements in decreasing order of their reactivity.
Sympathetic Vibratory Physics Electromotive Series
Which Metal Is More Active Than H2 Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. Many transition metals such as iron, chromium, nickel, tin, zinc, and lead are more reactive than hydrogen. The activity series is a list of elements in decreasing order of their reactivity. Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. Since metals replace other metals, while nonmetals replace. 26 rows metals that require the loss of only one electron to form stable ions are more reactive than similar metals which require the loss of. The most active metals are so reactive that they readily combine with the o 2 and h 2 o vapor in the atmosphere and are therefore stored under an inert liquid, such as mineral oil. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the. A reactivity or activity series is the arrangement of metals from most reactive to.
From slideplayer.com
Reaction Prediction What you MUST know before you even begin trying to Which Metal Is More Active Than H2 Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the. Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. A reactivity or activity series is the arrangement of metals from most reactive to. Since metals replace other metals, while nonmetals. Which Metal Is More Active Than H2.
From www.mdpi.com
Metals Free FullText MagnesiumBased Sacrificial Anode Cathodic Which Metal Is More Active Than H2 Since metals replace other metals, while nonmetals replace. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the. Many transition metals such as iron, chromium, nickel, tin, zinc, and lead are more reactive than hydrogen. The activity series is a list of elements in decreasing order of their. Which Metal Is More Active Than H2.
From www.adda247.com
Reactivity series of Metals and Non Metals Which Metal Is More Active Than H2 Since metals replace other metals, while nonmetals replace. 26 rows metals that require the loss of only one electron to form stable ions are more reactive than similar metals which require the loss of. A reactivity or activity series is the arrangement of metals from most reactive to. The activity series is a list of elements in decreasing order of. Which Metal Is More Active Than H2.
From www.pinterest.co.uk
MetalReactivitiesSeries Fe iron, Science blog, Metal Which Metal Is More Active Than H2 Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. A reactivity or activity series is the arrangement of metals from most reactive to. The activity series is a list of elements in decreasing order of their reactivity. The most active metals are so reactive that they readily combine with the o 2. Which Metal Is More Active Than H2.
From www.slideserve.com
PPT Chapter 8 Chemical Reactions PowerPoint Presentation, free Which Metal Is More Active Than H2 The activity series is a list of elements in decreasing order of their reactivity. 26 rows metals that require the loss of only one electron to form stable ions are more reactive than similar metals which require the loss of. The most active metals are so reactive that they readily combine with the o 2 and h 2 o vapor. Which Metal Is More Active Than H2.
From triptonkosti.ru
Тип химической связи h2 схема образования 86 фото Which Metal Is More Active Than H2 26 rows metals that require the loss of only one electron to form stable ions are more reactive than similar metals which require the loss of. Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. Since metals replace other metals, while nonmetals replace. The activity series is a list of elements in. Which Metal Is More Active Than H2.
From www.slideserve.com
PPT Reactivity Series of Metals PowerPoint Presentation, free Which Metal Is More Active Than H2 Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. The activity series is a list of elements in decreasing order of their reactivity. The most active metals are so reactive that they readily combine with the o 2 and h 2 o vapor in the atmosphere and are therefore stored under an. Which Metal Is More Active Than H2.
From www.nagwa.com
Question Video Determining Which Metal Is the Most Active Nagwa Which Metal Is More Active Than H2 Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the. The activity series is a list of elements in decreasing order of their reactivity. A reactivity or activity series is the arrangement of metals from most reactive to. Since metals replace other metals, while nonmetals replace. Many transition. Which Metal Is More Active Than H2.
From fphoto.photoshelter.com
science element postassium Fundamental Photographs The Art of Science Which Metal Is More Active Than H2 The most active metals are so reactive that they readily combine with the o 2 and h 2 o vapor in the atmosphere and are therefore stored under an inert liquid, such as mineral oil. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the. Since metals replace. Which Metal Is More Active Than H2.
From www.vrogue.co
The Activity Series Pathways To Chemistry vrogue.co Which Metal Is More Active Than H2 A reactivity or activity series is the arrangement of metals from most reactive to. 26 rows metals that require the loss of only one electron to form stable ions are more reactive than similar metals which require the loss of. The most active metals are so reactive that they readily combine with the o 2 and h 2 o vapor. Which Metal Is More Active Than H2.
From www.iccons.com.au
The Insight Galvanic Corrosion and Fasteners ICCONS Which Metal Is More Active Than H2 The activity series is a list of elements in decreasing order of their reactivity. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the. Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. The most active metals are so reactive. Which Metal Is More Active Than H2.
From www.chegg.com
Solved In Part I of the experiment, a piece of zinc metal is Which Metal Is More Active Than H2 The activity series is a list of elements in decreasing order of their reactivity. Many transition metals such as iron, chromium, nickel, tin, zinc, and lead are more reactive than hydrogen. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the. A reactivity or activity series is the. Which Metal Is More Active Than H2.
From selftution.com
Reactivity Series of Metals and Nonmetals » Selftution Which Metal Is More Active Than H2 A reactivity or activity series is the arrangement of metals from most reactive to. The activity series is a list of elements in decreasing order of their reactivity. Since metals replace other metals, while nonmetals replace. The most active metals are so reactive that they readily combine with the o 2 and h 2 o vapor in the atmosphere and. Which Metal Is More Active Than H2.
From slideplayer.com
Redox and Electrochemistry ppt download Which Metal Is More Active Than H2 The most active metals are so reactive that they readily combine with the o 2 and h 2 o vapor in the atmosphere and are therefore stored under an inert liquid, such as mineral oil. 26 rows metals that require the loss of only one electron to form stable ions are more reactive than similar metals which require the loss. Which Metal Is More Active Than H2.
From www.nagwa.com
Question Video Identifying Which Metal Can React with Acid and Its Which Metal Is More Active Than H2 Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the. Many transition metals such as iron, chromium, nickel, tin, zinc, and lead are more reactive than hydrogen. The most active metals. Which Metal Is More Active Than H2.
From canvids.com
Experiment brass, copper, or steel Which metal is stronger Canvids Which Metal Is More Active Than H2 Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the. The activity series is a list of elements in decreasing order of their reactivity. 26 rows metals that require the loss of only one electron to form stable ions are more reactive than similar metals which require the. Which Metal Is More Active Than H2.
From www.slideserve.com
PPT Predicting Products The Activity Series & Solubility Rules Which Metal Is More Active Than H2 A reactivity or activity series is the arrangement of metals from most reactive to. Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the. Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. Many transition metals such as iron, chromium,. Which Metal Is More Active Than H2.
From www.chegg.com
Solved Single Displacement Reactions Report Form Which Metal Is More Active Than H2 Calcium and the metals that are more reactive than calcium in the reactivity series can react with cold water to form the. The activity series is a list of elements in decreasing order of their reactivity. The most active metals are so reactive that they readily combine with the o 2 and h 2 o vapor in the atmosphere and. Which Metal Is More Active Than H2.